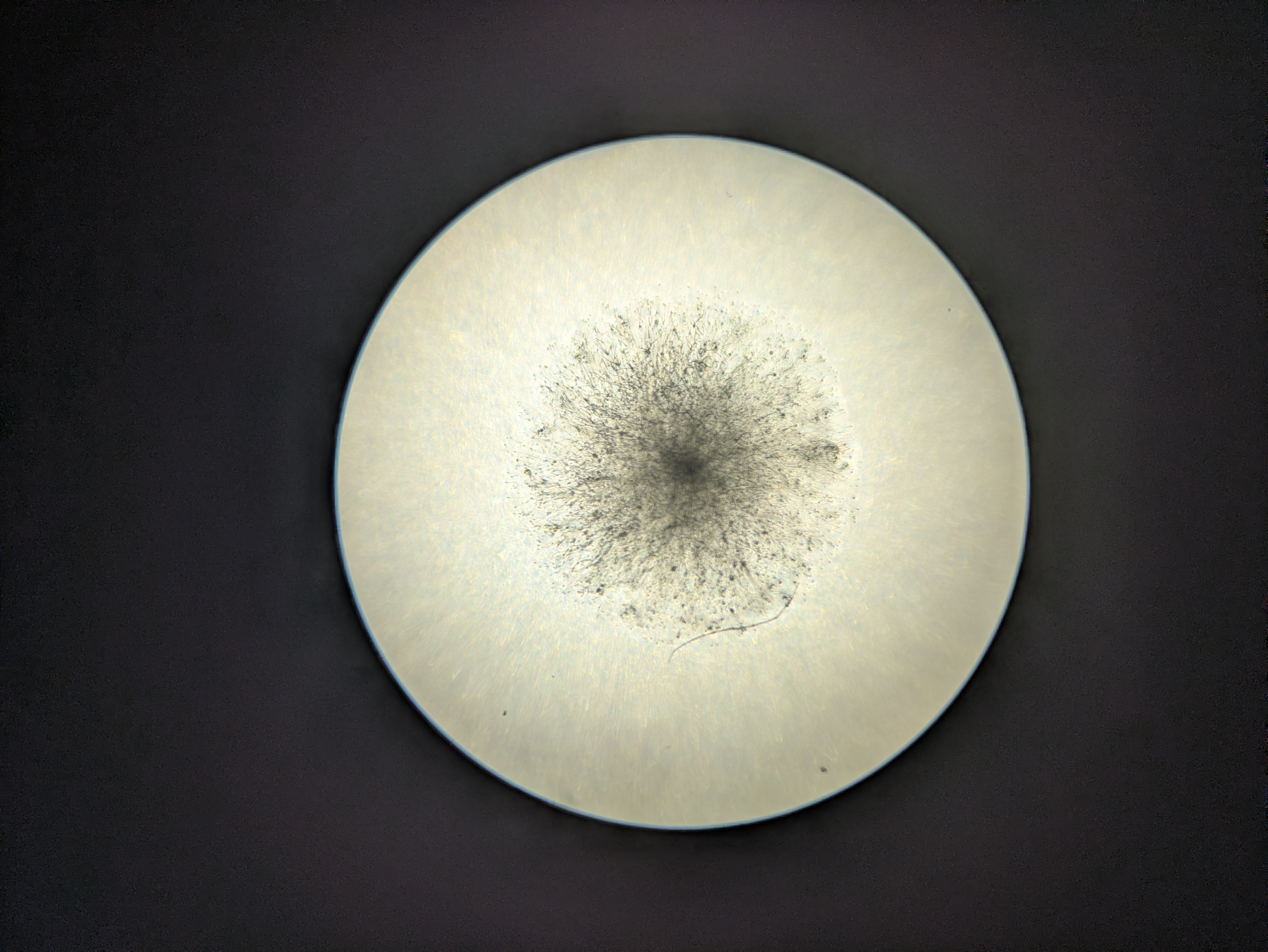
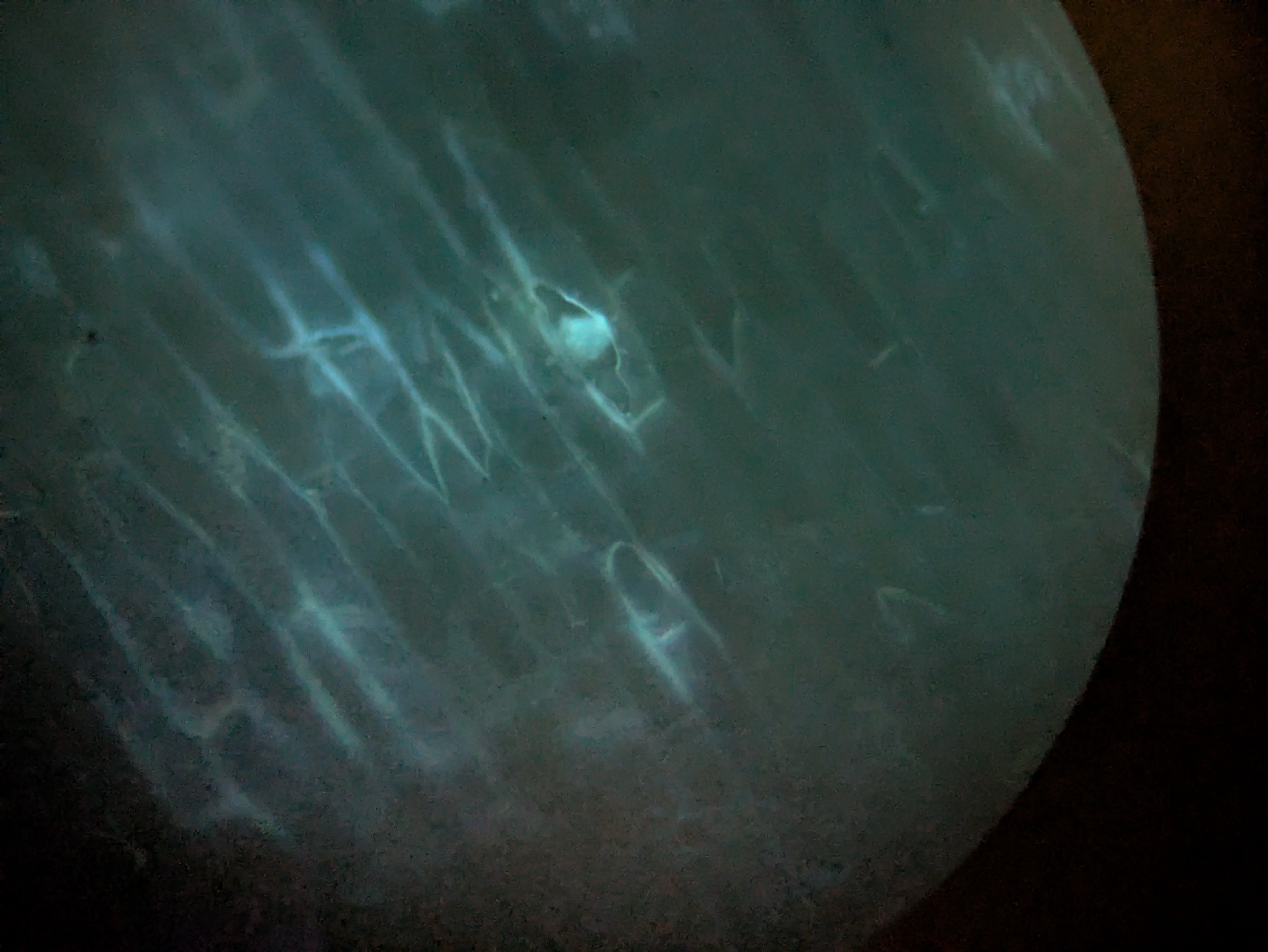
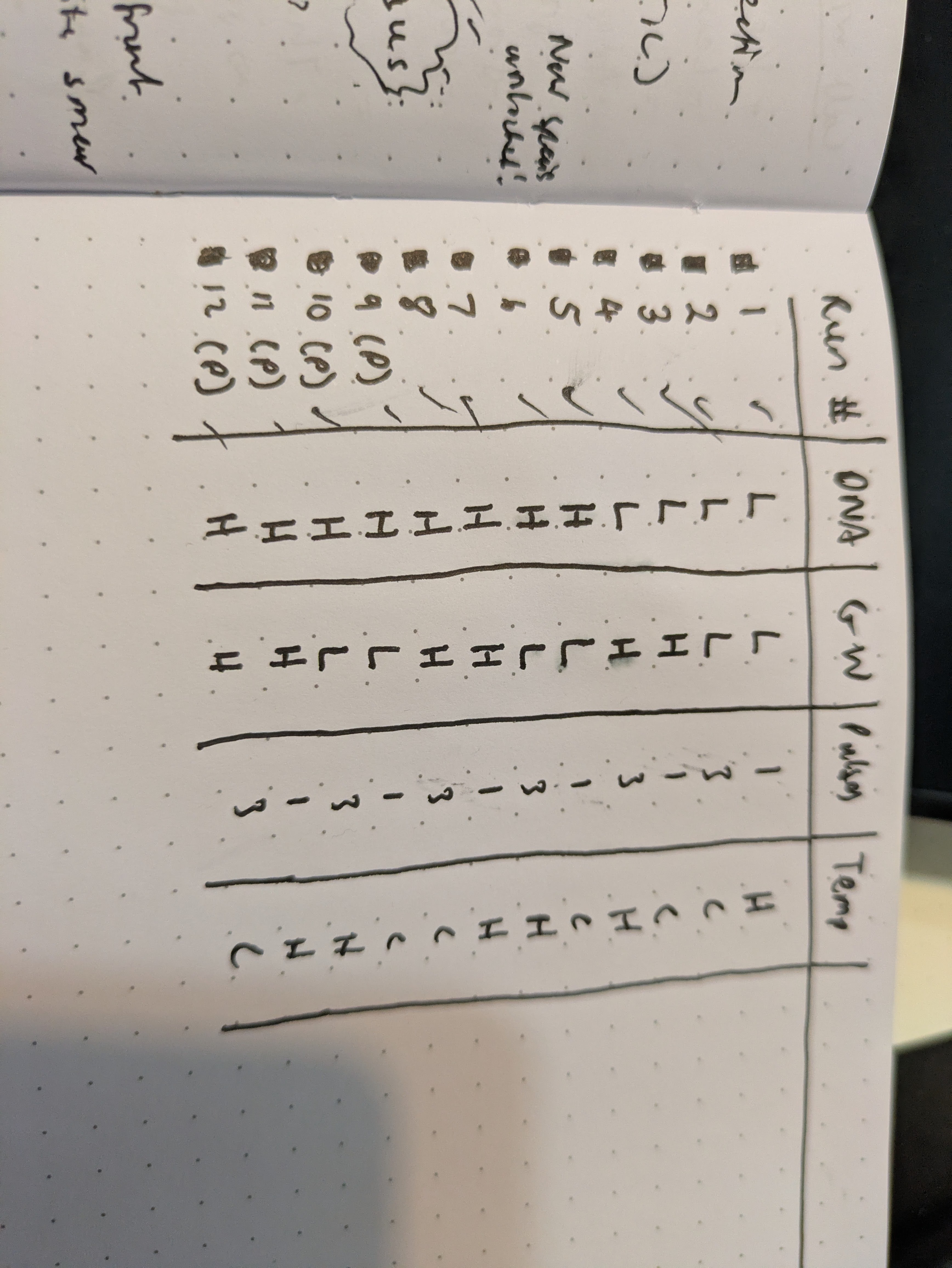
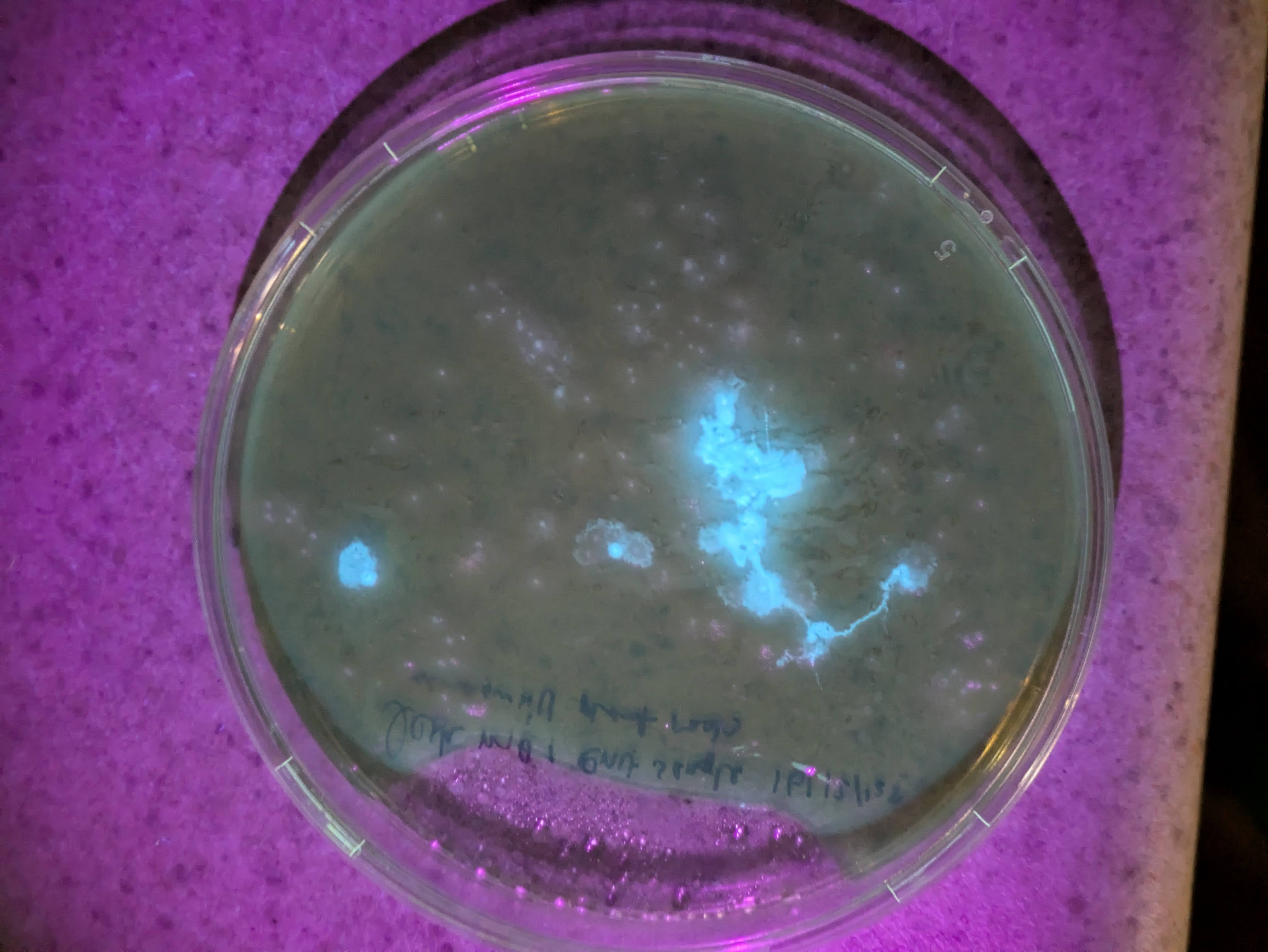
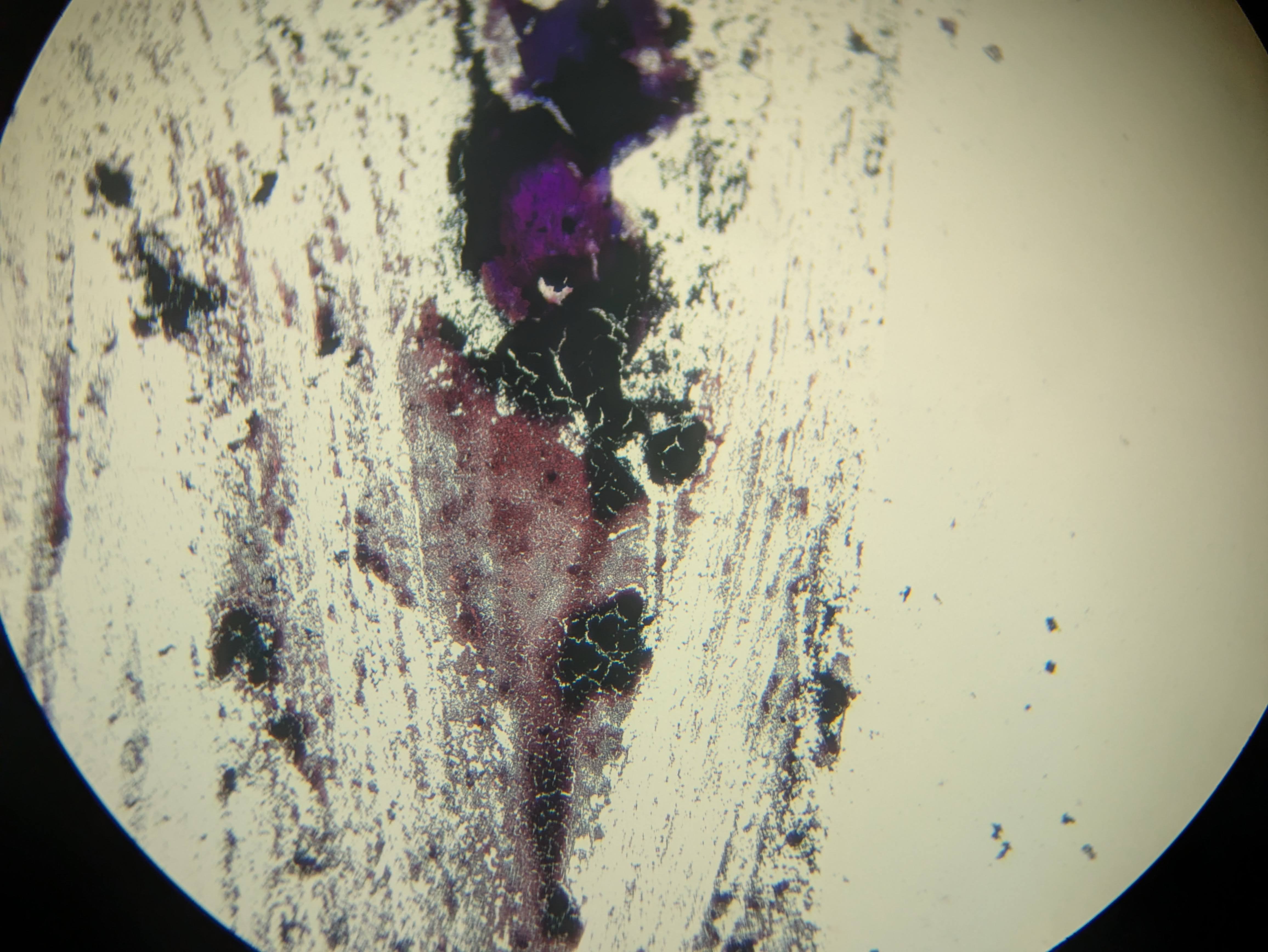
I got excited when I noticed that the flowers which I had injected with the Odin's agrobacterium Ruby system had some bright fluorescence under 360 nanometer UV light around the injection sites. There was a little bit of red ruby expression too, as I expected, but nowhere had I seen anything about a fluorescent reporter. This was exciting because I had used some DNA that I had extracted via miniprep from the agrobacterium in a first YOLO test of my Gene gun. I rushed to fish the test onion slices out of the dustbin and put them under the scope with a UV light illuminating them. Sure enough on the positive sample that I had shot, I could see some vague blue glow in a few cells similar to the pictures I saw online for the original O'Reilly Jean gun instructions. Very exciting! Except that I didn't know why they should be fluorescent genes in this plasma. I asked the Odin people and they confirmed that it shouldn't have any fluorescence- it's just Ruby. I am now tasting on a fresh flower, just the injection media or just water to see if the fluorescence is a response to damage by the primrose plant- my suspicion is that the onion was also some sort of autofluorescence and unrelated to the DNA that we shot into it. Update: yup, just the injection media was enough to cause some bright fluorescence in the petals - false alarm.